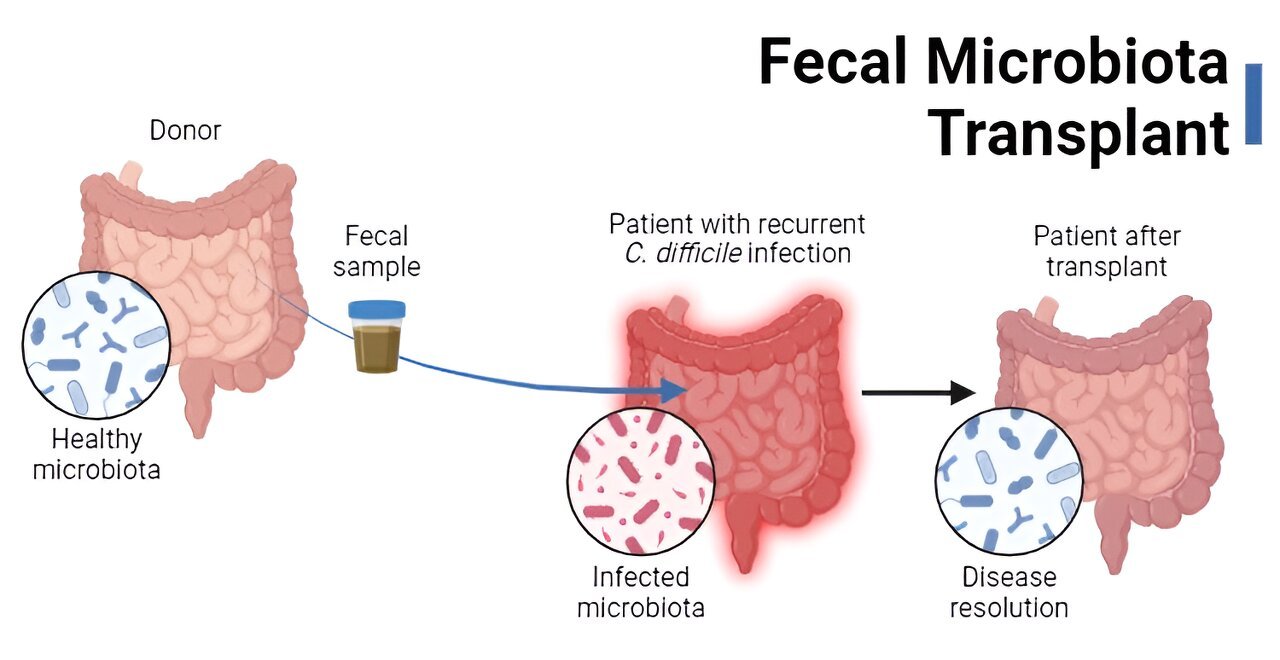
The premise of fecal microbiota transplants (FMT) is, admittedly, not the most pleasant. The process involves transferring donor stool (or derivatives thereof) to a recipient for a therapeutic purpose—namely, to restore the microbiota to a state capable of resisting the gut pathogen Clostridioides difficile.
But where did the idea for FMTs even come from? What’s new in the field of transferring feces—and what does the future hold?
It began with feces soup
The birth of FMTs precedes knowledge of microbes, much less the gut microbiota. The first records date back to 4th century China, where “yellow soup” (i.e., human fecal slurry) was used to treat patients with severe diarrhea and food poisoning. Though quite possibly the worst soup ever, it was reported to have “brought patients back from the brink of death.”

Fast forward to 1958, when the first “modern” FMT was performed. In this case, fecal enemas were used to cure 4 patients with pseudomembranous colitis, likely caused by C. difficile. Normally, the gut microbiota resists colonization by C. difficile. However, if the microbiota is disrupted, typically following antibiotic treatment for another infection, the pathogen can survive and thrive—secreting toxins, causing diarrhea and damaging the intestine.
The researchers in the 1958 study knew that antibiotics presumably killed off gut microbes and hypothesized that reintroducing “normal” bacteria into the gut via the fecal enemas would “re-establish the balance of nature.” The practice wasn’t deliberately used for C. difficile infection (CDI) until the 1980s, with some success.
Yet, the treatment never took off. Krishna Rao, M.D., M.S., the co-founder and director of the Fecal Microbiota Transplantation program at the University of Michigan, points to a couple of reasons why. For one, there was the “ick” factor—the idea of administering a slurry of feces to people via enema wasn’t very appealing to patients or practitioners.
Second, “CDI was a much different disease back then,” he said. “It was viewed as a nuisance, not a massive threat and burden on the health care system.” (These days, C. difficile causes nearly half a million infections and 30,000 deaths in the U.S. every year). While antibiotics are the standard treatment for CDI, in some cases they don’t work, and the infection will come back (recur), leading to a vicious and potentially deadly cycle of disease.
According to Rao, realization of the full threat of CDI in the 2000s—and increasing rates of recurrent disease—helped bring FMT into the light. But a “landmark” 2013 trial exploring the efficacy of FMT for recurrent CDI gave the practice legs.
The trial showed that infusion of donor stool was incredibly effective at treating patients with recurrent CDI, compared to vancomycin administration alone (94% versus 31% cure rate, respectively). In fact, it was so effective that the study was stopped early, and FMTs were administered to patients in other treatment arms because not doing so was considered unethical.
Later that year, the U.S. Food and Drug Administration (FDA) announced it would exercise enforcement discretion when FMT was used to treat recurrent CDI. That is, patients would not need to receive treatment through a clinical trial, as would be required if FMTs were used for other conditions. This announcement made FMT more accessible to physicians and, ultimately, CDI patients.
Citation:
Fecal microbiota transplants: Past, present and future (2024, February 9)
retrieved 9 February 2024
from https://medicalxpress.com/news/2024-02-fecal-microbiota-transplants-future.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.