Scientists have discovered that a protein linked to prostate cancer is associated with more aggressive disease—it could be a new target for treatment and be used to help predict who will become resistant to hormone therapy.
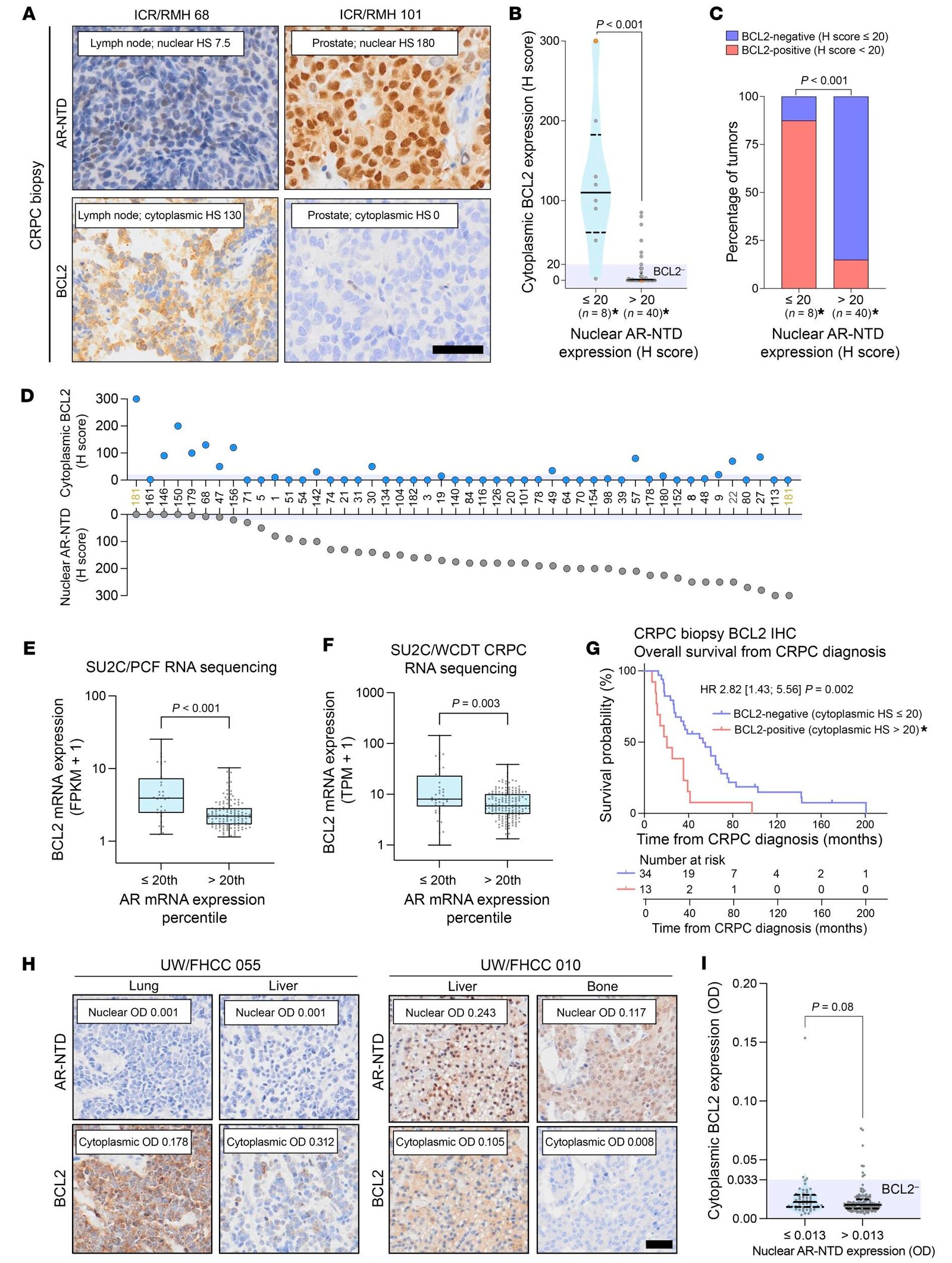
In new research published in the Journal of Clinical Investigation, a team at the Institute of Cancer Research, London, showed that the BCL2 protein was abundant in a subtype of advanced prostate cancer which has stopped responding to hormone therapy. The researchers estimate that about 10% of people with prostate cancer have high levels of this protein.
The team proposed a new treatment strategy to target BCL2—a protein responsible for preventing cell death. A clinical trial for prostate cancer testing the BCL2 inhibitor venetoclax—a drug approved for types of leukemia—with the hormone therapy enzalutamide has already begun.
Patients with high levels of BCL2 have a shorter overall survival
Metastatic castration-resistant prostate cancer (mCRPC) is an advanced type of cancer which remains fatal, and new treatments are urgently required. In one of the largest studies of its kind, the researchers examined 427 biopsies from 245 patients with mCRPC in two independent cohorts.
They found that patients with higher levels of BCL2 had a significantly shorter overall survival from the time of diagnosis of mCRPC, of 20.4 months compared with 53.0 months.
In the second cohort, they found a significant difference in response to common hormone therapy treatments, depending on the cancer’s level of BCL2. The PSA level—a marker of prostate cancer—fell by more than 50% in only 12.5% of those with higher levels of BCL2, compared with 47.6% of patients with lower expression of BCL2. The overall survival from starting therapy was also markedly different, at 9.7 months for those with higher levels of BCL2 compared with 24.3 months.
Discovering potential alternative treatments
However, the researchers found no difference in overall survival or PSA response when cancers were treated with the chemotherapy docetaxel, suggesting this could be a more beneficial treatment than hormone therapy for patients with high levels of BCL2.
In the lab, the team attempted to target BCL2. They found the best anti-tumor response in cells when they targeted the BCL2 “family” of proteins together—BCL2, BCLXL and MCL1—due to the interactions between these proteins in the body. The researchers suggest that in humans, drug delivery technologies—such as antibody-drug conjugates—which target cancer cells specifically and spare healthy tissue, would be needed to safely target these three proteins together.
‘Large disparity in outcomes’
Dr. Adam Sharp, Leader of the Translational Therapeutics Group at The Institute of Cancer Research, London, and Honorary Consultant Medical Oncologist at The Royal Marsden NHS Foundation Trust said, “We urgently need new treatments to help improve the quality and quantity of life for patients living with advanced prostate cancer.
“Our results have shown that there’s a large disparity in outcomes for people whose cancers have high levels of the BCL2 protein, and that their cancers respond less well to hormone therapies than others. Further research could provide evidence for more personalized treatment plans, as these cancers may respond better to docetaxel than enzalutamide or abiraterone.
“The researchers Daniel Westaby, Juan Jiminez-Vacas and Ines Figueiredo, played a critical role in understanding the connection between the BCL2 protein and this subtype of prostate cancer, as well as uncovering the regulatory mechanisms of the protein.”
Professor Johann de Bono, Professor of Experimental Cancer Medicine at The Institute of Cancer Research, London, and Consultant Medical Oncologist at The Royal Marsden NHS Foundation Trust said, “BCL2 is a protein that promotes cell survival, and we have shown that cancers with higher levels of the protein cause significantly worse outcomes for patients.
“If targeting BCL2 proves effective in clinical trials, patients with advanced prostate cancer will be able to look forward to better, personalized treatments. Our research has also suggested a combination strategy for therapies which should be explored and may prove even more effective than targeting BCL2 alone.”
‘Finding ways to tackle treatment resistance is essential’
Professor Kristian Helin, Chief Executive of The Institute of Cancer Research, London, said, “We know that while a drug may keep one person’s cancer at bay for a long time, unfortunately it may quickly stop working in another person. Finding ways to tackle treatment resistance—and to provide alternatives earlier on—is essential.
“This research has helped to identify a subset of patients who might respond better to alternatives to hormone therapy, and who may benefit even further from a BCL2 inhibitor. I look forward to seeing tests which pick out these patients and am hopeful these findings will lead to new treatment strategies for patients with advanced prostate cancer.”
More information:
Daniel Westaby et al, BCL2 expression is enriched in advanced prostate cancer with features of lineage plasticity, Journal of Clinical Investigation (2024). DOI: 10.1172/JCI179998
Citation:
New drug target discovered for aggressive form of prostate cancer (2024, September 18)
retrieved 18 September 2024
from https://medicalxpress.com/news/2024-09-drug-aggressive-prostate-cancer.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.








