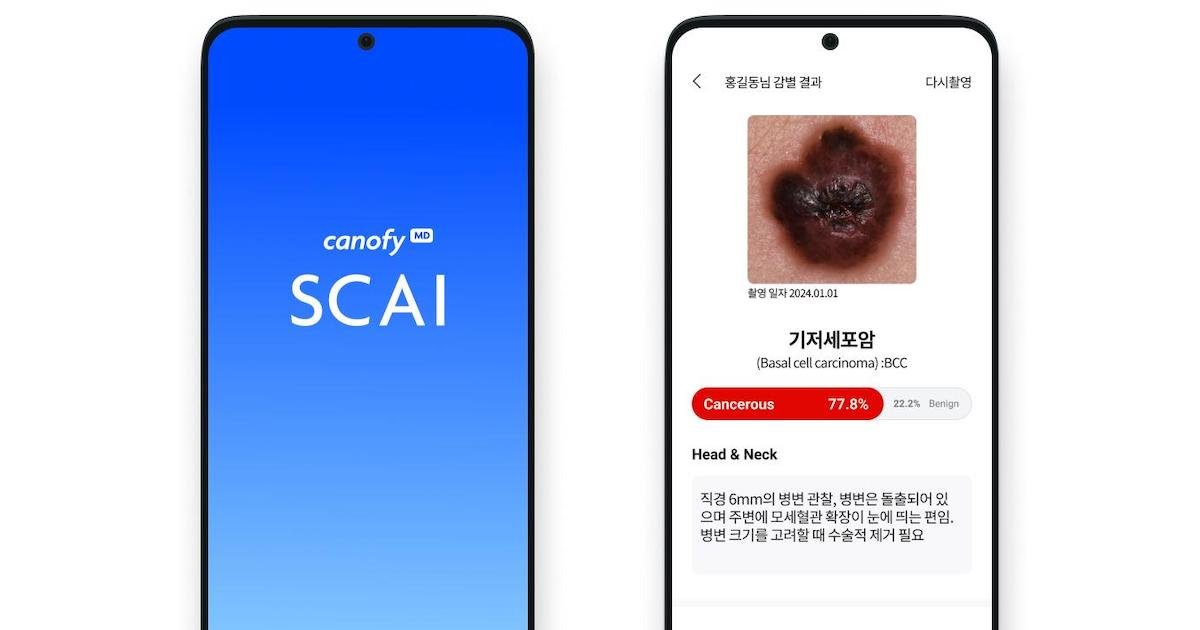
Korean digital health company LifeSemantics has obtained the first approval for an AI-powered solution for skin cancer diagnosis in South Korea.
The company received regulatory clearance from the Ministry of Food and Drug Safety for canofyMD SCAI four months after its application.
The mobile AI technology was developed through the Doctor Answer 2.0 project, which has gathered 30 hospitals and 19 technology companies to advance their medical AI development.
WHAT IT’S ABOUT

The flagship canofyMD solution uses convolutional neural network, an AI method for image recognition and processing, to detect and assist with the diagnosis of skin cancer in images taken from smartphones. It can identify malignant melanoma, basal cell carcinoma, and squamous cell carcinoma, as well as benign tumours.
In March, LifeSemantics completed the clinical trial of the mobile screening app, the first such trial for skin cancer AI in South Korea. The AI, which was trained using 6,500 images of skin lesions collected from three local hospitals, was trialled in 199 cases. It later showed “superior” results, including a diagnostic accuracy of 80.9%.
Following its regulatory clearance, LifeSemantics reportedly plans to further verify canofyMD SCAI in trials with six local hospitals. It previously disclosed that it targets to commercially launch the app by the last quarter of the year. It also looks to file for regulatory approvals in Australia and New Zealand, where skin cancer rates are one of the highest worldwide.
Furthermore, LifeSemantics will continue conducting clinical trials and pursuing similar regulatory approvals for its other canofyMD solutions, which include mobile software for blood pressure prediction, hypertension complication prediction, and hair density analysis.
WHY IT MATTERS
The number of people getting treated for skin cancers in South Korea has been increasing over the past years. Latest data from the Health Insurance Review and Assessment Service shows that the number of people with skin cancer grew by 34% between 2022 and 2018. Globally, new cases recorded each year top 1.5 million.
Demand for solutions to detect and prevent skin cancers early is driving the growth of the skin cancer diagnosis market, which is expected to be worth $5 billion in 2028 from $3 billion in 2021.
MARKET SNAPSHOT
Early this year in the United States, DermaSensor obtained approval from the Food and Drug Administration for its AI-enabled device for skin cancer detection. It is the first such device intended for primary care which can detect skin cancers with an accuracy high up to 96%.
Australia, which records one of the highest ultraviolet radiation levels and cases of skin cancers worldwide, is also home to innovations in skin cancer diagnosis. ASX-listed Advanced Human Imaging also offers a smartphone-based AI called DermaScan AI for classifying skin conditions based on photos. It can classify 588 common and rare skin conditions in 133 categories, including all categories of skin cancer.
A team of Australian eye experts are currently trying to adapt a desktop system for measuring UV radiation damage to the eye – a potential early indicator of skin cancer – into smartphones. It will also utilise AI algorithms which they said will work best for mobile software.
AI was also applied to assess and confirm cancerous lesions in images captured in a pop-up clinic project piloted last year. The project by Skin Check Champions, the University of South Australia, and The Hospital Research Foundation was launched during the 2023 Tour Down Under road cycling race.