In 1957, British oceanographer Anthony Laughton famously observed, “The deep oceans cover over two-thirds of the surface of the world, and yet more is known about the shape of the surface of the moon than is known about that of the bottom of the ocean.”1
Anna Klompen studies cnidarian venom systems and participates in outreach events to help others learn about these often underappreciated invertebrates.
Anna Klompen
Decades later, Anna Klompen came across this quote, which sparked her interest in marine biology. Eschewing the charismatic megafauna of the ocean, Klompen instead turned to the poorly understood cnidarians, a heterogenous phylum that includes corals, sea anemones, Hydra, box jellyfish, and true jellyfish. Cnidarians don’t have brains or strong muscles or sharp teeth, and most are slow or even sessile. Nevertheless, they can be formidable predators thanks to their dazzlingly complex venom systems.

Cnidarians produce a wide array of toxins, from sea anemone neurotoxins that act on sodium channels to the potently hemolytic toxins in box jellyfish.2,3 These creatures also administer their venom in an unusual manner: Specialized organelles called nematocysts are highly pressurized, touch-sensitive capsules that burst open with incredible speed to eject toxin-laden darts into any creature unfortunate enough to brush against them. Understanding the biomechanics of these capsules may inspire new microdevice designs for drug delivery and other applications.4,5
Klompen, now a postdoctoral researcher at the Stowers Institute for Medical Research, spoke about the genetics of jellyfish toxins, the biomechanics of stinging organelles, and how cnidarian studies can help scientists understand how cells construct their internal machinery.
What kinds of toxins are in cnidarian venoms?
Their venoms contain a variety of biomolecules that have different mechanisms of action. A lot of the drug discovery work using cnidarians has focused on sea anemones, including Nematostella vectensis. That’s because sea anemone venoms often contain many neurotoxins that target different ion channels with a high specificity. That’s ideal for a drug. We want it to target a specific channel, and we want it to perform a precise action.
Jellyfish venoms have fewer neurotoxins; instead, they have pore-forming toxins as well as different types of enzymes that break down proteins and lipids.6 That’s not as ideal for drugs, but some of these enzymes might be specific enough that they could be interesting to explore as molecular tools.
To build a more comprehensive picture of the many components in cnidarian venoms, researchers use known toxin genes from other species as a guide to look for “new” cnidarian toxins. However, many sequences in the genome look similar to those of toxins even though the protein that they encode performs another function.

Hydractinia, seen here growing on the edge of a glass microscope slide, are colonial animals in which polyps with different duties differentially express venom-like genes.
Anna Klompen
For example, when we analyzed tissue from an entire polyp from the species Hydractinia symbiolongicarpus, we found more than 150 venom-like genes in the transcriptome.7 In an animal like a snake, researchers might focus on the venom gland transcriptome to help narrow down which genes encode true venom toxins. However, cnidarians’ venom systems are not centralized in this way, making it difficult to determine which genes are actually important for venom.
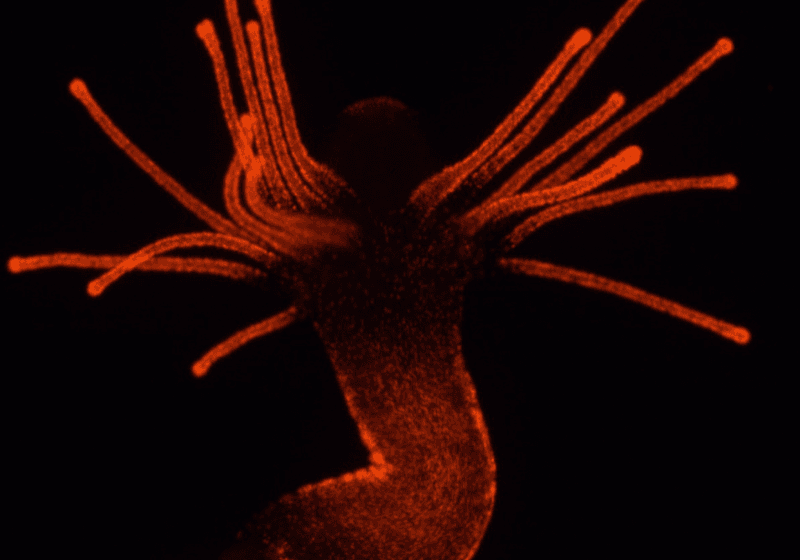
Hydractinia are colonial animals. They consist of many little genetically identical animals that are interconnected with tiny tubes. Through these tubes, they share food, stinging cell precursors, and even specific kinds of stem cells.8 They’re also modular. Within the colony, some individuals catch prey and eat while others just reproduce. I wanted to investigate which venom-like genes were expressed in the stinging cells and also whether a polyp’s “job” shaped its toxin profile, which might indicate that they use different toxins for different purposes.
To find out, I made a transgenic Hydractinia line that expressed a red fluorescent protein in stinging cells. Then using fluorescence activated cell sorting, we separated the stinging cells from the rest of the tissue and analyzed their transcriptomes. We found about 100 venom-like genes upregulated in the stinging cells. Several venom-like genes were differentially expressed between stinging cells in different polyp types, with greater numbers of venom-like genes upregulated in feeding polyps.7 This makes sense since these polyps might need more complex venoms to catch prey, whereas reproductive polyps only need venom for self-defence. Through studies like this one, we can start to understand how venom composition relates to its function.
How do cnidarians deliver their venoms?

An engineered juvenile Nematostella sea anemone expresses fluorescent green proteins in nematocysts.
Anna Klompen
Cnidarians are decorated in tens of thousands of tiny nematocysts produced by specialized stinging cells. Nematocysts can be anywhere from five to 15 microns in size, which is thinner than a sheet of paper. This is one of the only examples of a full-body venom system as opposed to a gland attached to fangs or spines.
The way a nematocyst injects its venom is really interesting. Nematocysts are made of collagen-like proteins that allow them to be super pressurized and able to fire extremely quickly. In one recording, the harpoon-like structure of a Hydra nematocyst accelerated to more than five million g in 700 nanoseconds.9 It could be days before they have the opportunity to fire these stinging cells into their unsuspecting prey, so they need to be able to hold that pressure for long periods of time.
The protein structures of the nematocysts are unique. They’re almost like little, hollow hypodermic needles with various kinds of barbs. From a biomechanical standpoint, they’re innovative. Cnidarians manufacture thousands to tens of thousands of these complex structures all the time, and we just don’t know how that process works. I am currently using a sea anemone stinging cell reporter line to learn more about how these structures are made, which may reveal important insights that can be used in future bio-inspired technological applications.
What can stinging cells teach scientists about fundamental intracellular processes?

In the larval form of a Nematostella sea anemone, fluorescent probes of different colors mark RNA transcripts of interest to identify different populations of stinging cells and indicate developmental stages.
Anna Klompen and Cathy McKinney
In my current research, I study these structures as a way to explore how cells build themselves and their internal structures. We often think of cells as the building blocks of life, but they are actually made of even smaller subunits. How do cells build complex organelles like nematocysts? The proteins that eventually make up these stinging organelles have some attributes of self assembly, and we know that the self-assembly and gene regulation processes that help build these structures work together in some way. However, teasing those two mechanisms apart is really difficult.
Because stinging cells are so specific and so numerous, they could be really good tools for answering those kinds of questions: How do cells build themselves? What’s the contribution of each of these assembly processes? And how could that help us understand other complex cellular features that we might see in other organisms?
This interview has been condensed and edited for clarity.
References
- Laughton AS. Exploring the Deep Ocean Floor.J R S Arts. 1957;106(5017):39-56.
- Sachkova MY et al. The Birth and Death of Toxins with Distinct Functions: A Case Study in the Sea Anemone Nematostella.Mol Biol Evol. 2019;36(9):2001-2012.
- Brinkman DL et al. Transcriptome and venom proteome of the box jellyfish Chironex fleckeri.BMC Genomics. 2015;16(1):407.
- Tal Y et al. Continuous Drug Release by Sea Anemone Nematostella vectensis Stinging Microcapsules.Mar Drugs. 2014;12(2):734-745.
- Karabulut A et al. The architecture and operating mechanism of a cnidarian stinging organelle.Nat Commun. 2022;13:3494.
- Remigante A et al. Impact of Scyphozoan Venoms on Human Health and Current First Aid Options for Stings.Toxins (Basel). 2018;10(4):133.
- Klompen AML et al. Venom system variation and the division of labor in the colonial hydrozoan Hydractinia symbiolongicarpus.Toxicon X. 2022;14:100113.
- Frank U et al. The hydroid Hydractinia: a versatile, informative cnidarian representative. Bioessays. 2001;23(10):963-971.
- Nüchter T et al. Nanosecond-scale kinetics of nematocyst discharge. Curr Biol. 2006;16(9):R316-R318.