Yale University researchers have discovered that glucose metabolism plays a critical role in guiding the early development of mouse embryos, revealing that specific metabolic pathways regulate essential cell signaling during key phases of embryogenesis.
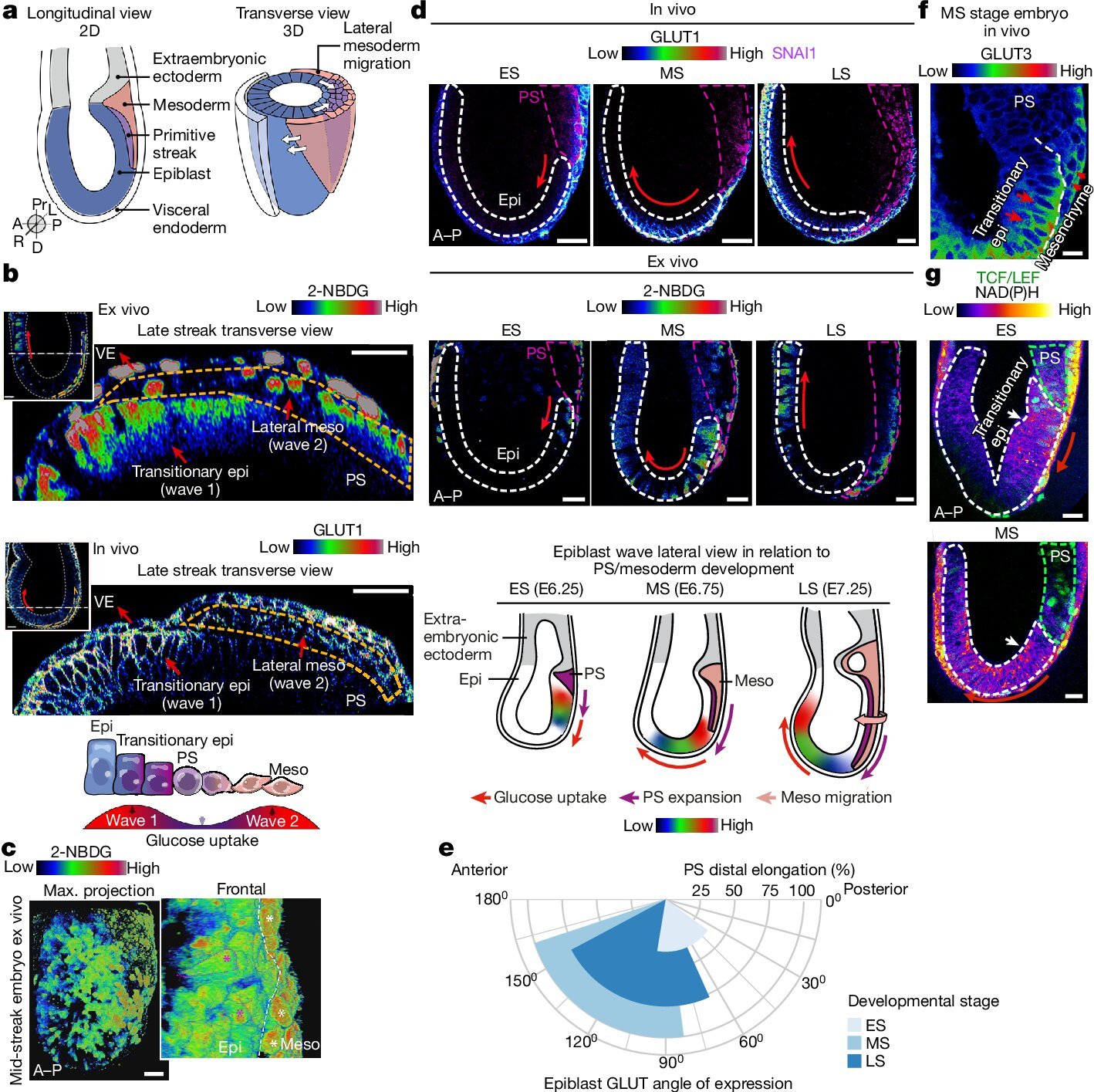
The study, “Selective utilization of glucose metabolism guides mammalian gastrulation,” published in Nature, identifies two distinct waves of glucose utilization during mouse gastrulation.
The first wave channels glucose through the hexosamine biosynthetic pathway (HBP) in epiblast cells, facilitating the formation of proteoglycans for fibroblast growth factor (FGF) signaling. This signaling is crucial for cell differentiation and the extension of the primitive streak. This structure eventually forms the neural plate, becoming the basis for the spinal cord and nervous system.
The second wave directs glucose through glycolysis, providing energy and metabolites necessary for mesodermal cell migration and lateral expansion.
The team mapped the spatial and temporal patterns of glucose uptake using single-cell-resolution imaging of developing mouse embryos, stem cell models, and embryo-derived tissues.
They found that the initial wave of glucose metabolism occurs in posterior epiblast cells and expands forward as the development progresses. The subsequent wave of glycolysis supports the movement of mesodermal cells away from the primitive streak, promoting lateral expansion.
To test the certainty of the pathway involvements, the team inhibited glucose metabolism through chemical blockers, experimentally confirming the disruption of the primitive streak formation and mesoderm specification.
Specifically targeting the HBP impaired the development of the primitive streak while inhibiting late-stage glycolysis with affected mesodermal cell migration without hindering initial cell fate decisions.
The study also demonstrates that glucose metabolism influences extracellular signal-regulated kinase (ERK) signaling pathways, finding them crucial for cell differentiation and movement during gastrulation. Inhibition of glucose metabolism led to reduced ERK activity, while supplementation with N-acetylglucosamine, a product of the HBP, restored ERK signaling and rescued developmental defects.
Further analysis using stem cell-based embryo models and mesoderm explants confirmed that the HBP is essential for epiblast cell fate transitions and that glycolysis supports the migratory behavior of mesodermal cells.
RNA sequencing of treated mesoderm explants revealed downregulation of pathways involved in cell migration and extracellular matrix interactions when glycolysis or ERK signaling was inhibited.
The findings highlight that glucose metabolism, in coordination with genetic and signaling mechanisms, is integral to the successful patterning and morphogenesis of the developing embryo. This research challenges the traditional view of cellular metabolism as merely a background cellular function, positioning it instead as an active director of embryonic development.
More information:
Dominica Cao et al, Selective utilization of glucose metabolism guides mammalian gastrulation, Nature (2024). DOI: 10.1038/s41586-024-08044-1
Christian Schröter, Glucose has a surprise role in directing cell fate and migration, Nature (2024). DOI: 10.1038/d41586-024-03284-7
© 2024 Science X Network
Citation:
Glucose metabolism drives embryonic development in mice, study reveals (2024, October 19)
retrieved 19 October 2024
from https://medicalxpress.com/news/2024-10-glucose-metabolism-embryonic-mice-reveals.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.