A new study that provides unprecedented insights into the chemical bonding of antimony could have a profound impact on materials research. The collaboration between scientists from Leipzig University, RWTH Aachen University and the DESY synchrotron in Hamburg combined experimental measurements with theoretical calculations.
The findings will help scientists to better understand phase change materials and, in particular, improve their application in data storage and thermoelectrics. The research has now been published in Advanced Materials.
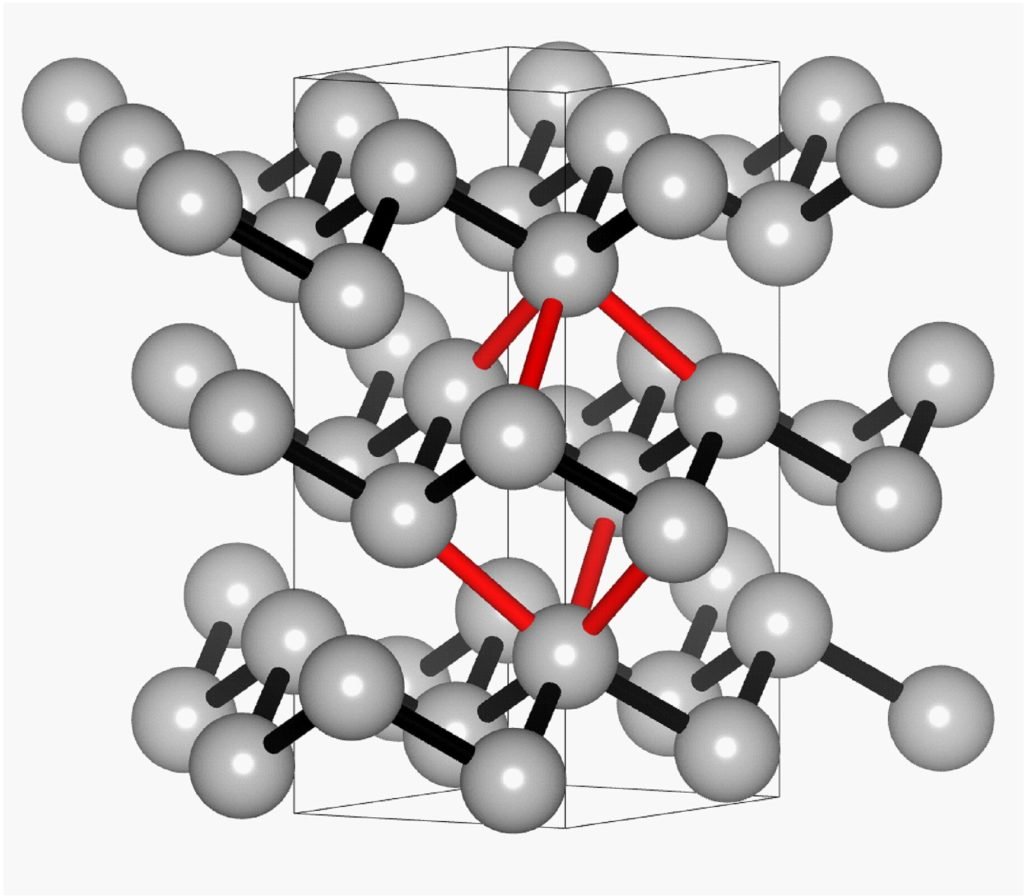
The study combined experimental measurements with theoretical calculations, with the aim of analyzing the nature and strength of the chemical bonding in antimony. “The strength of a bond depends directly on the distance between the atoms,” says Professor Claudia S. Schnohr of the Felix Bloch Institute for Solid State Physics at Leipzig University, adding that comparisons with other materials such as metals and semiconductors show that this distance dependence is characteristic of the type of chemical bond.
Of particular note is the demonstrably smooth transition between classical covalent bonding and electron-rich multi-center bonding. Covalent bonding is seen, for example, in semiconductors such as germanium. “Our results show that antimony in its stable phase exhibits characteristics of both types of bonding,” says co-author Professor Oliver Oeckler of the Institute of Inorganic Chemistry and Crystallography at Leipzig University.
This has important implications for the understanding of phase change materials, which are used in data storage and thermoelectrics, among other applications.
Antimony as a model for phase change materials
“We investigated antimony as an elemental model system for phase change materials. It has a similar structure to germanium telluride, but consists of only one type of atom,” says Professor Schnohr. These properties facilitate analysis and comparison with other materials to better understand their bonding characteristics.
The findings could be used to optimize material properties. “In the future, the experimental or theoretical determination of force constants will make it possible to tailor the design of new materials,” says Schnohr.
More information:
Franziska Zahn et al, Experimental and Theoretical Force Constants as Meaningful Indicator for Interatomic Bonding Characteristics and the Specific Case of Elemental Antimony, Advanced Materials (2025). DOI: 10.1002/adma.202416320
Citation:
Antimony’s bonding characteristics offer insights into phase change materials (2025, January 22)
retrieved 22 January 2025
from https://phys.org/news/2025-01-antimony-bonding-characteristics-insights-phase.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.