A group of scientists from IOCB Prague, led by Dr. Miloslav Polášek, have created compounds that are up to a million times more stable than similar substances used in contemporary medicine to treat tumors or as contrast agents for magnetic resonance imaging.
They have found a new way to securely bind metal elements, known as lanthanides, inside molecules of medical drugs. Their study has been published in the journal Nature Communications.
“Lanthanides are indispensable in medicine, yet there is a continual challenge to enhance their binding strength within pharmaceutical molecules. Progress in this area has stagnated for the last thirty years. Stability is crucial, particularly for gadolinium-containing contrast agents used in magnetic resonance imaging. This is important because gadolinium can cause damage to the body if it escapes from the drug molecule,” Dr. Polášek points out.
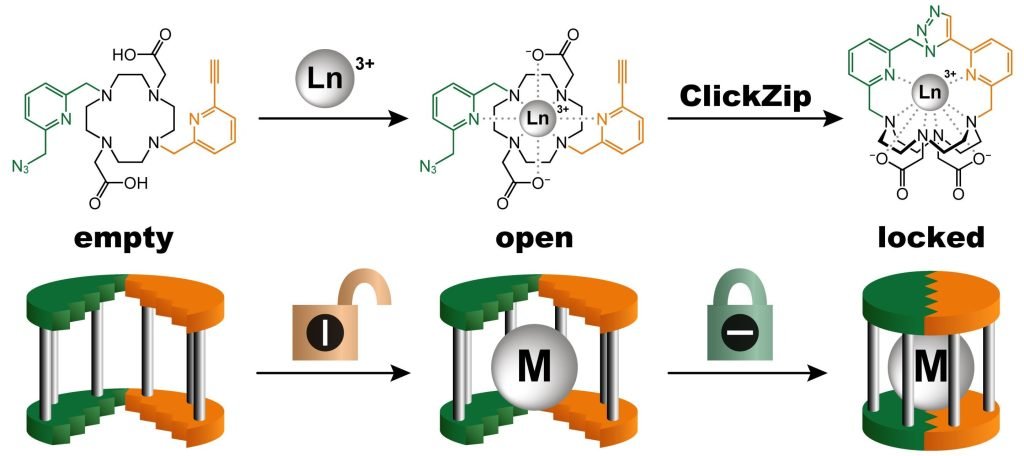
“Our new synthetic principle called ‘ClickZip’ incorporates these metals into molecules virtually irreversibly, making them much safer to use.”
“ClickZip functions as a trap. Initially, the molecule is open, allowing the metal to easily enter a prepared binding site. Once it is seated there, the ‘trap door’ irreversibly closes behind it through a chemical reaction, ensuring the metal cannot escape. It is elegant and beautiful chemistry,” says Dr. Tomáš David from IOCB Prague, the first author of the publication.
“A million times greater stability means that the compound lasts a million times longer. It is like comparing the length of one TV series episode with the length of a human life.”
The new compounds are expected to benefit, for example, people with impaired kidney function, who cannot undergo some magnetic resonance imaging examinations. This is because their bodies excrete the contrast agents only slowly, thus increasing the risk that they might release the toxic element gadolinium. However, the potential applications do not end here.
ClickZip molecules are so stable that they can literally be boiled in concentrated acid without decomposition. With these compounds it is possible to tag, for example, molecules of a peptide drug for testing in an animal model. Thanks to such specific tags, it is possible to precisely and sensitively determine which tissues the drug has reached and in what amount.
“We can even use several tags simultaneously and thus study multiple parameters within a single organism. This allows us to collect more data and to spare many experimental animals,” says Polášek, emphasizing the ethical implications of the research.
The feasibility of this in vivo multiplexing has been demonstrated by the researchers in collaboration with Dr. Lenka Maletínská, who leads the research on peptide-based anti-obesity drugs at IOCB Prague.
This breakthrough discovery made at IOCB Prague broadens the horizons not only of medicine, but also of modern branches of industry. The authors have patented the technology and are now looking for a partner that would help them bring it to the market.
The research was carried out thanks to the CarDia project (LX22NPO5104) under the EXCELES program, which aims to strengthen social resilience to the impacts of serious diseases.
More information:
Tomáš David et al, Ultra-inert lanthanide chelates as mass tags for multiplexed bioanalysis, Nature Communications (2024). DOI: 10.1038/s41467-024-53867-1
Citation:
A molecular trap for exotic metals promises improved diagnostics and faster drug development (2024, November 25)
retrieved 25 November 2024
from https://phys.org/news/2024-11-molecular-exotic-metals-diagnostics-faster.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.







